Image source: Biogen
This month, the world of Alzheimer’s has been rocked by lecanemab, the treatment developed by American biotech company Biogen with Japanese pharmaceutical company Eisai. It’s a positive step, but there is more to be done. Research suggests that quantum devices may be able to assist future efforts to discover drug treatments for diseases that have, to this point, vexed scientists.
Lecanemab
On 6th January, the FDA approved lecanemab for the treatment of early Alzheimer’s disease in the US. Biogen has now announced that it is submitting marketing authorization for lecanemab in Europe. If lecanemab is approved by the regulators that govern the UK, it would be the first new Alzheimer’s drug here for nearly 20 years. Lecanemab completed phase 3 clinical trials in late 2022, slowing the progression of Alzheimer’s in people with early onset of the disease. In an 18-month study that enrolled nearly 1,800 people worldwide, patients who took lecanemab showed a 27% slower decline in cognitive function than those who had a placebo.
Caution has been advised though. According to Dr Posteinsson, Director of the University of Rochester Alzheimer’s Disease Care Research and Education Program (AD-CARE), the new drug is not for everyone suffering from Alzheimer’s.
“Alzheimer’s is a very complex, multi-modal disease that varies from person to person. This is also a disease where the changes in the brain start maybe 20 to 25 years before you have any clinical symptoms. By the time someone presents with symptoms they are actually very late in the overall disease course”.

– Dr Posteinsson, Director of the University of Rochester Alzheimer’s
Disease Care Research and Education Program (AD-CARE)
Although not life-threatening, about 12.5 percent of participants in the trials showed evidence of mild to moderate localized brain swelling. There was also a higher rate of micro haemorrhages, or pinhead bleeds, that are often seen in patients with Alzheimer’s disease. The consensus is that the treatment requires careful clinical and MRI monitoring, particularly in the first 6-12 months.
Cause of Alzheimer’s
A leading cause of Alzheimer’s is the spread of plaques throughout the brain, killing neurons and decreasing brain function. One such plaque causing Alzheimer’s is formed by the build-up and aggregation of a protein called Amyloid-β (Aβ peptides). Lecanemab works by dissolving the Amyloid-β plaques in the brain. Amyloid-β is produced and released by another protein called Amyloid precursor protein (APP). Amyloid precursor protein crosses nerve cell membranes and is cleaved on either side of the membrane, releasing chains of amino acids. Under certain conditions, however, rather than a healthy chain of amino acids being released, Amyloid-β is produced and released instead.
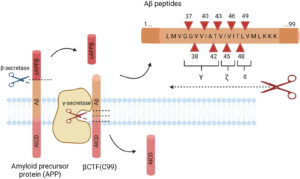
Image source: cellandbioscience.biomedcentral.com
Many other organisations are continuing to conduct pioneering drug development for Alzheimer’s by targeting an enzyme which is known to catalyse the release of Amyloid-β. The enzyme is called β-secretase. Ever since β-secretase was identified as a significant drug target for Alzheimer’s back in 2000, the common strategy for most pharmaceutical firms has been the use of an inhibitor. An inhibitor is a molecule that binds to an enzyme and blocks its activity. Inhibitors covering many different structural classes have been designed and developed to prevent the release and subsequent build-up of Amyloid-β. The same inhibitors however, have, so far, also caused significant side effects. For example, by also binding to other receptors, the inhibitors can prevent the production and release of proteins required for healthy brain function. It turns out that β-secretase is essential for the healthy development, repair and maintenance of the nervous system. Furthermore, some studies have shown that, in a healthy brain, a certain amount of Amyloid-β helps neurons strengthen their synapses to compensate for other neurons that have been silenced. The process is called homeostatic synaptic plasticity and is required for healthy brain activity.
“Amyloid-β is probably one of the driving forces of Alzheimer’s disease, but definitely not the only one. Tau, neuro-inflammation, and oxidative stress all play a role. This is a disease that will ultimately require a combination of treatments, like some of the resistant cancers, or resistant high blood pressure, or diabetes. Those of us who have been studying Alzheimer’s for a long time have become humble and we recognize that lecanemab is a start and not a cure. It is a modest start but represents an approach to treatment that we can build upon”.
– Dr Posteinsson, Director of the University of Rochester Alzheimer’s
Disease Care Research and Education Program (AD-CARE)
The current thinking then, is that Amyloid-β does not represent the whole story. Most experts agree that there is much more to be done. There is an incredibly complex group of conditions involved in Alzheimer’s. β-secretase remains an important target for drug development, but rather than eradicating it entirely, ways to regulate levels of β-secretase are being considered, along with other approaches like tackling inflammation and blood vessel health within the brain.
Implications for Quantum
An important capability in the search for an effective Alzheimer’s treatment is the ability to accurately predict how potential β-secretase inhibitor molecules will bind to the target proteins as well as avoid binding to other non-target proteins. Back in October 2021, UK-based Cambridge Quantum and Roche, the Swiss multinational healthcare firm, published an excellent joint paper on “Quantum Computational Quantification of Protein-Ligand Interactions”. They used quantum computers to rank-order a series of β-secretase inhibitor molecules using their binding energy differences. Cambridge Quantum is widely recognised as being the first to demonstrate a hybrid classical and quantum computational approach to the problem of quantifying protein binding interactions. They used superconducting transmon (IBM) and trapped-ion (Honeywell) Noisy Intermediate Scale Quantum (NISQ) computers. Their approach combines the Density Matrix Embedding Theory (DMET) with the Variational Quantum Eigensolver (VQE) approach for finding molecular electronic ground states. It was an exploratory study and an important first step, successfully demonstrating the use of hybrid quantum and classical computation to a real-world application. There are limitations, but Cambridge Quantum and Roche concluded that, if appropriate error mitigation techniques are applied, and the system simplified sufficiently, NISQ hardware is capable of making significant contributions.
Since then, many collaborations between quantum computing companies and biotech companies have been announced. For example, in October 2022, IBM and Cleveland Clinic announced the deployment of the first private sector onsite IBM-managed quantum computer in the US. As part of a ‘Discovery Accelerator’ and supported by a $500 million investment from the State of Ohio, the deployment is anticipated to be complete this year (2023). Alzheimer’s is one of the areas of interest; and research projects include a quantum computing method for screening and optimizing drugs targeted to specific proteins.
There have been no claims that quantum computing played any part in the drug discovery or lead optimisation processes for Biogen’s lecanemab, although, like all major pharmaceutical companies, Biogen has invested in quantum computing research and development. Back in June 2017, Biogen announced the results of their quantum computing collaboration with Accenture Labs and the Canadian quantum computing software company, 1Qbit. At the time, Govinda Bhisetti, Head of Computational Chemistry at Biogen said: “Collaborating with researchers at Accenture Labs and 1QBit made it possible to rapidly pilot and deploy a quantum-enabled application that has the potential to enable us to bring medicines to people faster.” Biogen concluded that quantum-enabled methods have the potential to speed up drug discovery for diseases such as Alzheimer’s and Parkinson’s. They predicted that, “as quantum computers become more sophisticated and scale to larger, more connected qubits, perhaps as soon as 2018, they would be in a position to immediately harness the technology to speed up drug discovery”.
We are still not at the point where quantum computing, or even hybrid classical and quantum computational approaches, are driving large scale, drug discovery or lead optimisation. It’s still a long way off. Predicting when quantum computing will deliver is possibly the largest “make or break” question in quantum computing today. What the Cambridge-Roche demonstration showed us though, is that progress can be made, not only by improving the noise mitigation and fault tolerance of the hardware, but also by refining the model, for example, by expanding the quantum computation to include atoms of the binding site. Their paper sheds light on the hardware and software requirements required for applying NISQ algorithms to drug design. The groundwork has now been laid for future development of a productive workflow for computer-aided drug design in the NISQ era.
The positive results of Biogen’s lecanemab shows that reducing Amyloid-β can be a major part of the solution for Alzheimer’s. This also validates the wider approach to developing β-secretase inhibitor molecules, which was the focus of the Cambridge-Roche collaboration. The goal is clear, with Alzheimer’s disease now contributing to around 60-70% of the 55 million people living with dementia worldwide. There are nearly 10 million new dementia cases every year and it is the seventh leading cause of death among all diseases as well as one of the major causes of disability and dependency among older people. The physical, psychological, social and economic impact of Alzheimer’s, not only for patients, but also for their carers, families and society at large can be utterly overwhelming.
So, congratulations to Biogen and all those in the Alzheimer’s community. We hope and expect this will be the first of many positive steps in the fight against Alzheimer’s. As Dr. Ruoyi Zhou, Director of IBM Research for the Cleveland Clinic Partnership put it, “a step change in the way we solve scientific problems is on the horizon”.
If you found this article to be informative, you can explore more current quantum news here, exclusives, interviews, and podcasts.